Medical device makers find solution to FDA demands - Tooling and Production Magazine for Large Plant Management and Metalworking

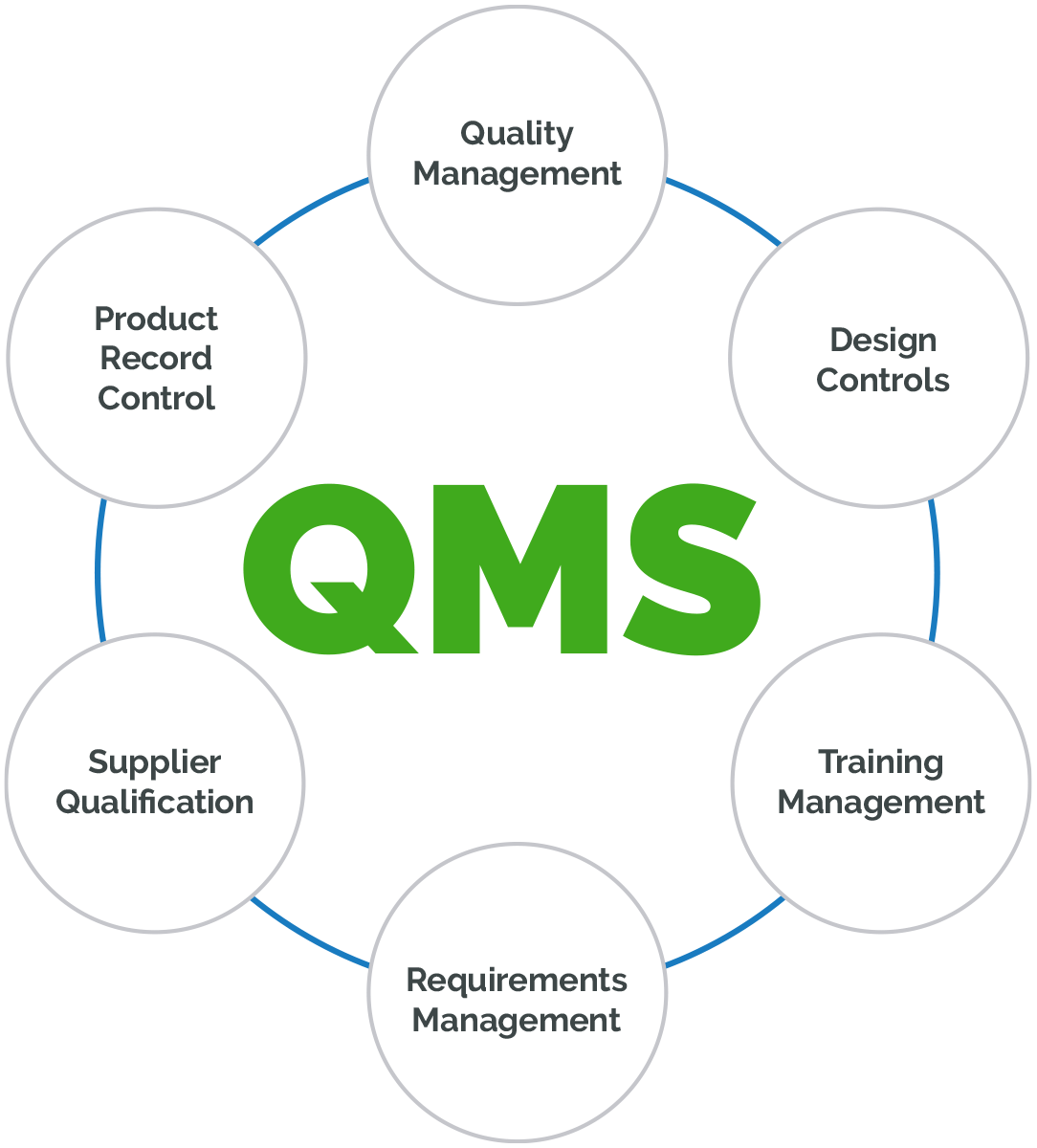
The FDA publishes its Quality Management System Regulation (QMSR) final Rule to harmonize with ISO13485 - Evnia

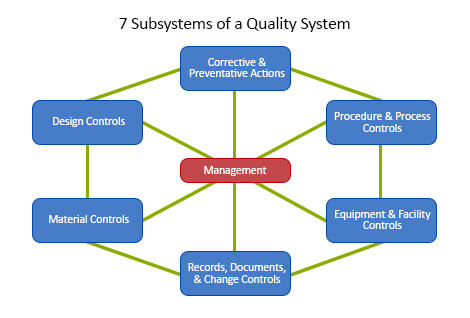
FDA Quality System Regulation for Medical Devices (21 CFR Part 820): A Practitioner's Guide to Management Controls (English Edition) eBook : Daugherty, D: Amazon.fr: Boutique Kindle

New FDA Quality Management System Regulation (QMSR): Why Medical Device Manufacturers Need to Prepare Now - Johner Institute New Zealand

Designing A World-Class Quality Management System For FDA Regulated Industries: Quality System Requirements (QSR) For cGMP : Muchemu, David: Amazon.fr: Livres

FDA 510(k) Consulting Service for Medical Devices » ISO 13485 Quality Management System Certification

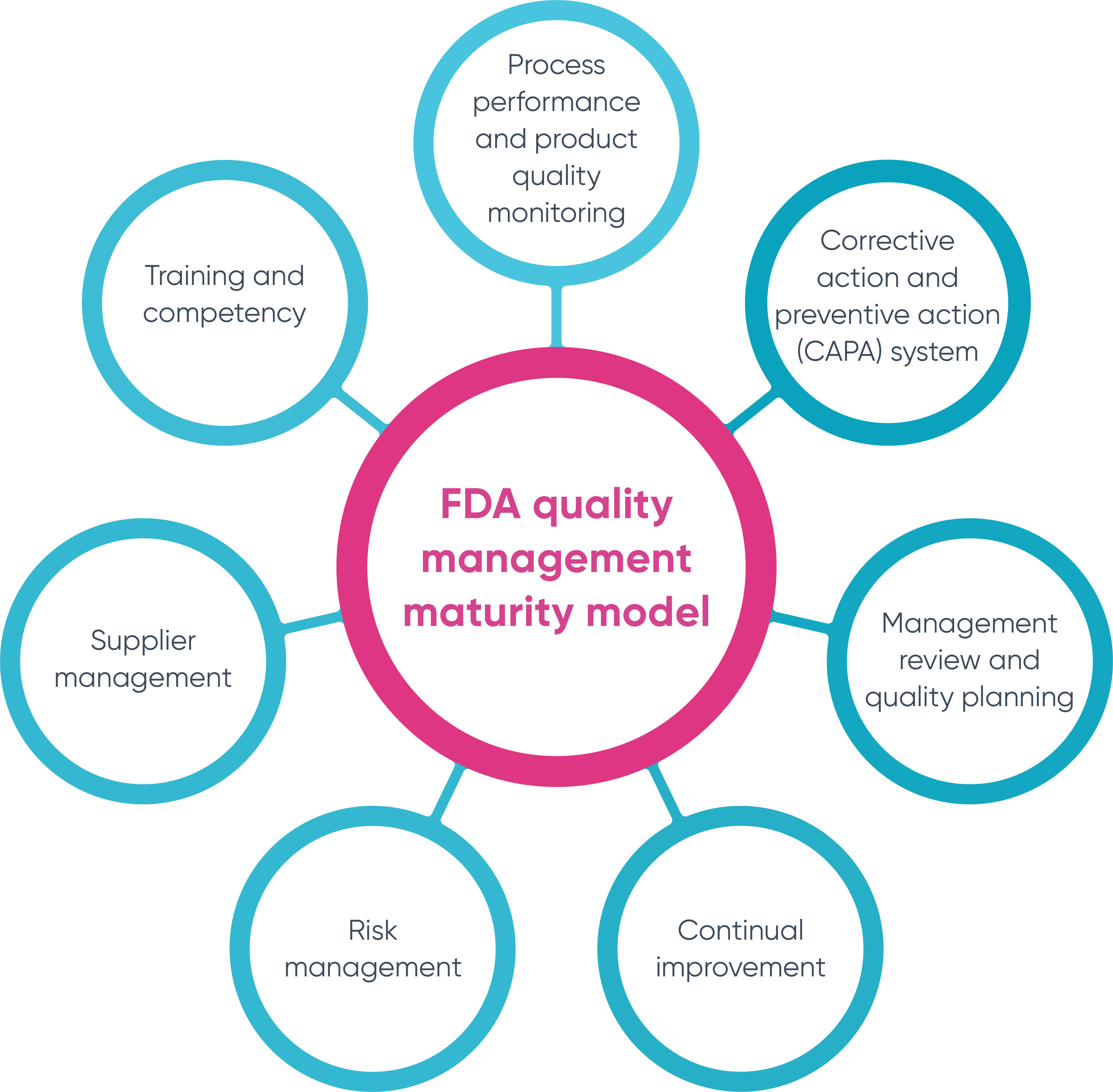
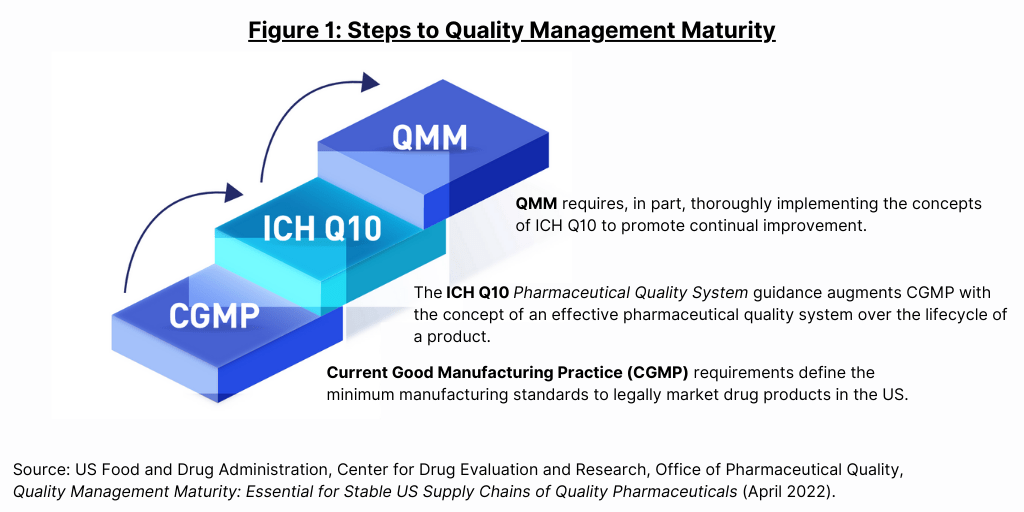
Live from PDA/FDA: FDA Considers Incentivizing Quality Management Maturity (QMM) | Healthcare Packaging
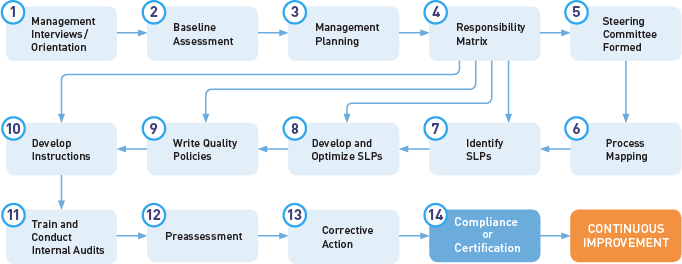
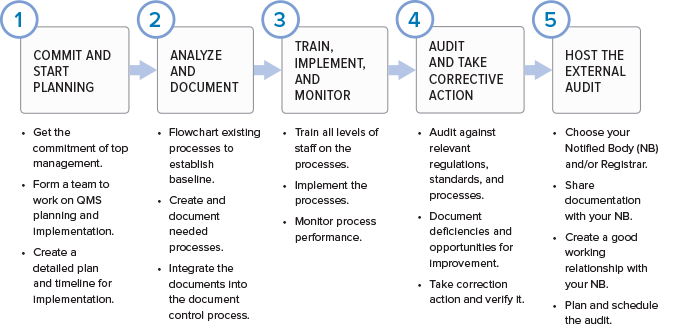
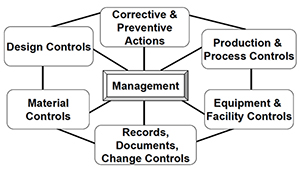
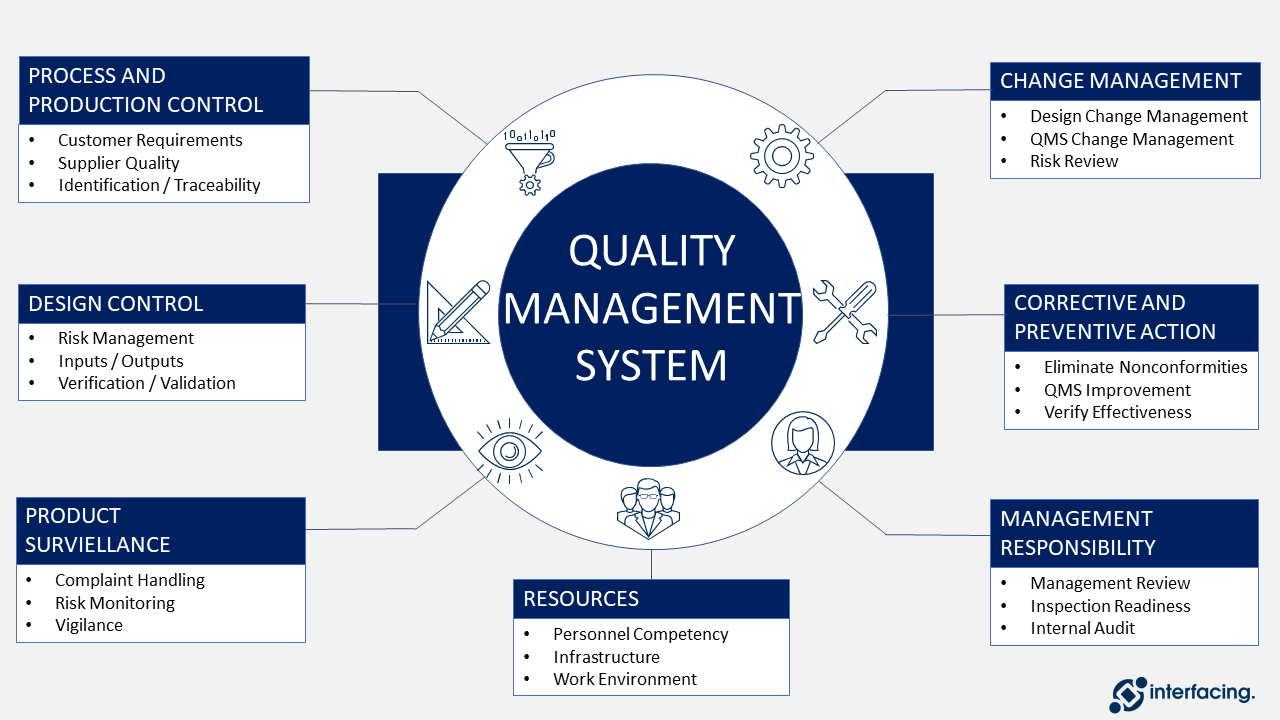
Understanding What Happens During a Medical Device QMS Inspection – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog